Are you deciding between electroplating and anodizing for your next project? Both methods improve metal surfaces but work differently and serve unique purposes. Let’s break it down so you can choose the best option for your needs.
Electroplating adds a thin layer of metal onto a surface using electricity. It enhances appearance, prevents corrosion, and improves wear resistance. On the other hand, anodizing creates a protective oxide layer on metals like aluminum. It boosts durability and allows for color dyeing. Each method has its strengths, depending on your project goals.
Let’s explore how these processes work, their benefits, and where they fit best. By the end, you’ll know which one suits your project.

Electroplating: Definition and Process
Electroplating adds a thin metal coating to a base metal surface. This process makes parts stronger, more durable, and resistant to corrosion.
What is Electroplating?
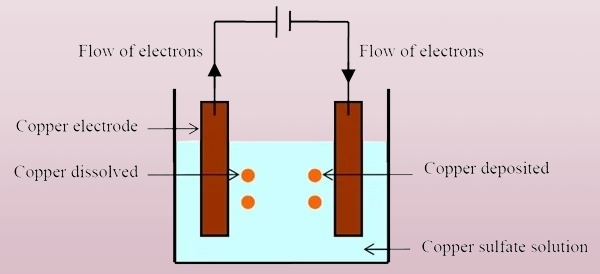
Electroplating is an electrochemical process in which a thin layer of metal is deposited onto the surface of an object. It is done using electrical current, which causes metal ions in a solution to bond to the surface of a substrate, typically a metal. This method is used to enhance the base material’s appearance, durability, and corrosion resistance.
Purpose of Electroplating
Electroplating serves several purposes. It can make a surface more corrosion-resistant, enhance its look, or improve electrical conductivity. It’s also used to build up worn parts or prepare surfaces for further processing.
How Electroplating Works?
Electroplating relies on an electric current to transfer metal ions from a solution onto a surface. The object to be plated acts as the cathode, while the coating metal serves as the anode. When electricity flows, metal ions bond to the object’s surface.
Step-by-Step Process of Electroplating
- Cleaning: The object is cleaned to remove dirt, grease, or oxides. This ensures the coating adheres properly.
- Preparation: The object is rinsed and sometimes treated with chemicals to improve plating quality.
- Plating: The object is submerged in a solution containing metal ions. An electric current is applied, causing the ions to bond to the surface.
- Rinsing and Drying: The plated object is rinsed to remove excess solution and then dried.
Types of Electroplating Techniques
Different metals are used for plating, depending on the desired outcome. Here are some common types:
Chromium Plating
Chromium or chrome plating provides a shiny, durable, and corrosion-resistant finish. It’s often used for decorative purposes, like on car parts or bathroom fixtures.
Copper Plating
Copper plating improves electrical conductivity and is often used as a base layer for other plating processes. It’s common in electronics and circuit boards.
Gold Plating
Gold plating provides a luxurious finish and excellent corrosion resistance. It’s used in jewelry, electronics, and high-end connectors.
Nickel Plating
Nickel plating is one of the most widely used electroplating techniques. It enhances corrosion resistance and provides a smooth, shiny surface. It’s commonly used in automotive and industrial applications.
Anodizing: Definition and Process
Anodizing changes a metal’s surface into a durable oxide layer, helping it resist corrosion and wear.
What is Anodizing?
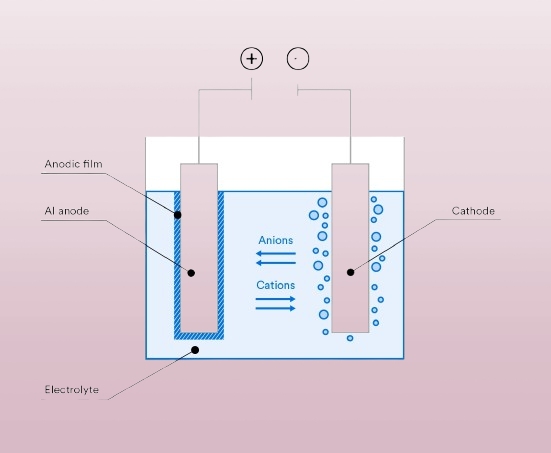
Anodizing is an electrochemical process that enhances the natural oxide layer on the surface of metals, especially aluminum. This process thickens the oxide layer, making it more durable, corrosion-resistant, and aesthetically pleasing.
Unlike electroplating, anodizing doesn’t add a metal coating but builds up the natural oxide layer already on the material.
Purpose of Anodizing
Anodizing improves a metal’s surface properties. It enhances durability, improves corrosion resistance, and allows for color customization.
The Anodizing Process Explained
Anodizing uses an acid bath and electricity to form an oxide layer. The metal part becomes the anode (positive), while a cathode plate completes the circuit. The process controls the oxide layer’s thickness and properties.
Step-by-Step Process of Anodizing
- Cleaning: The metal is cleaned to remove dirt, grease, or other contaminants.
- Pre-Treatment: The metal may be etched or brightened to prepare the surface.
- Anodizing: The metal is submerged in an acid electrolyte bath, and an electric current is applied, forming the oxide layer.
- Dyeing (Optional): The porous oxide layer can absorb dyes to add color.
- Sealing: The surface is sealed to lock in the dye and improve corrosion resistance.
Types of Anodizing Process
Anodizing works best on metals like aluminum, titanium, and magnesium. These metals naturally form oxide layers, making them ideal for the process.
Chromic Acid Anodization
This method uses chromic acid to create a thin, corrosion-resistant layer. It’s often used in aerospace applications where weight and durability are critical.
Anodizing with Sulfuric Acid
The most common method is sulfuric acid anodizing. This method produces a thicker oxide layer, making it suitable for decorative and industrial uses.
Hard Coat or Hard Anodize
Hard anodizing creates an extremely durable and wear-resistant surface. Creates the thickest, hardest coating. It’s used in high-stress applications like military equipment or industrial machinery.
Key Differences Between Electroplating and Anodizing
These two processes create different results through distinct methods. Here’s what sets them apart.
Structural and Chemical Differences
Electroplating adds a layer of another metal onto the surface using electricity. On the other hand, anodizing thickens the natural oxide layer of the metal itself. Electroplating changes the surface composition, while anodizing modifies the existing material.
Durability and Corrosion Resistance
Anodizing creates a harder, more wear-resistant surface compared to electroplating. The oxide layer formed during anodizing is highly resistant to corrosion. Electroplating can also improve corrosion resistance, but it depends on the type of metal used for plating.
Aesthetic Impact
Electroplating provides a metallic finish, which can be shiny or matte, depending on the metal used. Anodizing allows for color dyeing, offering a wider range of aesthetic options. However, electroplating can achieve a more reflective, mirror-like finish.
Thickness and Surface Texture Variations
Electroplating typically results in a thinner coating, often measured in microns. Anodizing creates a thicker oxide layer, which can be adjusted based on the application. The surface texture after anodizing is usually more porous, while electroplating produces a smoother finish.
Advantages of Electroplating and Anodizing
Both processes offer specific benefits for metal parts. Here’s what each method does best.
Advantages of Electroplating
Enhanced Conductivity and Adhesion
Electroplating can improve a part’s electrical conductivity by applying a thin layer of metal. This is especially useful for components like circuit boards that require high performance.
Electroplated layers bond well to the base material, ensuring the coating stays in place even under stress.
Cost-Effectiveness
Because the coating is thin, it uses less material. Electroplating is more affordable than other surface treatments for projects where appearance or a small layer is needed.
Versatility in Metal Finishing
Electroplating offers great versatility. It can be used with various metals, such as gold, chrome, nickel, and copper. This makes it suitable for many applications, from decorative finishes to enhancing the durability of parts.
Advantages of Anodizing
Increased Durability and Scratch Resistance
Anodized surfaces are much harder than untreated metals. This makes them more resistant to scratches and wear.
Aesthetic Benefits (Coloring and Appearance)
Anodizing also provides aesthetic benefits. The process works especially well with aluminum. The oxide layer is porous, allowing it to absorb dyes. This means anodized surfaces can be colored in vibrant, long-lasting shades.
Environmental Impact and Sustainability
Anodizing is considered more eco-friendly than electroplating. It does not require hazardous chemicals like those used in electroplating, and anodized aluminum is fully recyclable, making it a more sustainable option.
Comparing the Applications of Electroplating vs Anodizing
Both processes serve specific industries with their unique benefits. Let’s look at where each works best.
Electroplating Applications in Electronics and Automotive
The electronics industry uses:
- The gold plating on connectors
- Copper plating for circuit boards
- Silver plating for high-frequency parts
- Nickel plating for EMI shielding
Automotive sector needs:
- Chrome plating for trim pieces
- Zinc plating on fasteners
- Nickel plating for engine parts
- Copper plating under other finishes
Anodizing in Aerospace and Architectural Projects
Aerospace parts use anodizing for:
- Aircraft body panels
- Wing components
- Interior fittings
- Fasteners and brackets
Architectural uses include:
- Window frames
- Door handles
- Handrails
- Building panels

Which Method is Better for Specific Applications?
Each method works best for certain uses. Picking the right one saves time and money.
When to Choose Electroplating
Electroplating is a good choice when you need a metal coating for better conductivity, corrosion resistance, or wear protection. It’s perfect for parts that need a thin, smooth, shiny surface.
Electronic components like connectors and switches, automotive trim pieces, and jewelry are common uses. Electroplating is also ideal for coating a part with a specific metal.
When to Choose Anodizing
Anodizing improves hardness, corrosion resistance, and surface appearance. It’s especially effective for aluminum parts exposed to rough conditions or heavy handling.
Industries like aerospace and architecture often use anodizing for components that need extra durability and a polished look. The process creates a tough oxide layer that resists scratches.
Similarities between Electroplating and Anodizing
Both electroplating and anodizing are electrochemical processes that improve the performance and appearance of metals. They help enhance parts’ durability and corrosion resistance, making them suitable for use in harsh environments.
Both methods involve electricity to create a protective layer on the metal’s surface. While electroplating adds a metallic coating, anodizing builds up the natural oxide layer.
Conclusion
Electroplating and anodizing are popular surface treatment methods but serve different purposes. Electroplating adds a metallic coating to a surface, improving conductivity, corrosion resistance, and appearance. Anodizing thickens the natural oxide layer on metals like aluminum, making them more durable, scratch-resistant, and corrosion-resistant.
Need help deciding which method is right for your project? Contact us for expert advice and get a free quote tailored to your needs! We’re here to help you make the best choice for your manufacturing needs. Contact us today!
Hey, I'm Kevin Lee

For the past 10 years, I’ve been immersed in various forms of sheet metal fabrication, sharing cool insights here from my experiences across diverse workshops.
Get in touch

Kevin Lee
I have over ten years of professional experience in sheet metal fabrication, specializing in laser cutting, bending, welding, and surface treatment techniques. As the Technical Director at Shengen, I am committed to solving complex manufacturing challenges and driving innovation and quality in each project.