Stainless steel is strong and resists rust, but can still fail in certain conditions. Electrolytic corrosion often surprises engineers and manufacturers when stainless steel loses its strength. The problem worsens in harsh environments, where weak spots appear and lower performance. These weak spots can also shorten the service life of parts.
Engineers need to understand the science behind electrolytic corrosion. They should know what causes it, how to prevent it, and how to repair it. This knowledge helps them make better choices when designing and using stainless steel.

What Is Stainless Steel Electrolytic Corrosion?
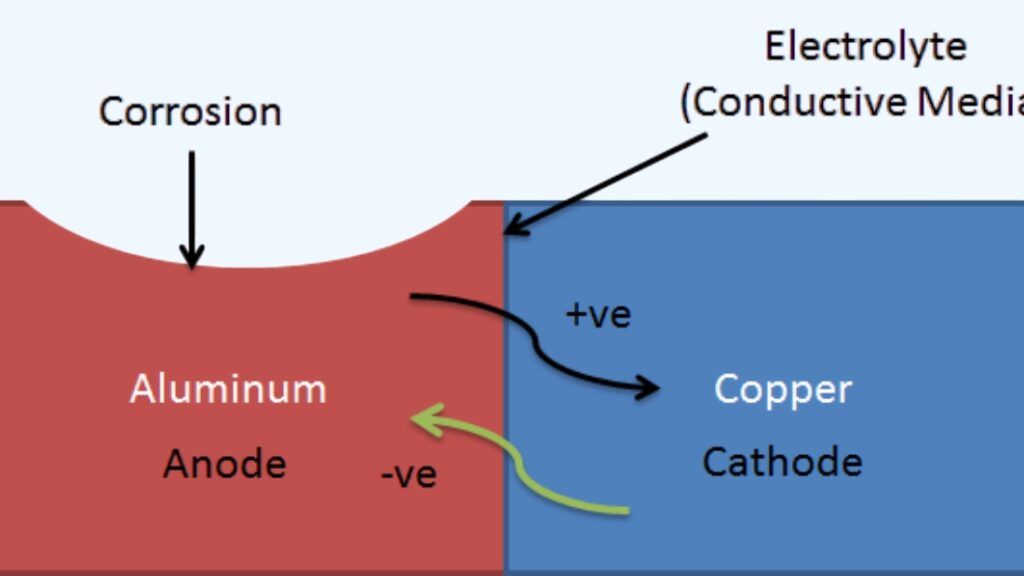
Electrolytic corrosion happens when stainless steel breaks down under an electrical current in a wet or conductive setting. The current can come from contact with other metals, stray electricity, or charged particles in the environment. When this occurs, electrons move between different areas on the metal, creating an anode and a cathode. The anode loses material, which shows up as pits, cracks, or surface thinning.
This process is not the same as normal rusting. Rusting can happen in air and moisture, but electrolytic corrosion needs an electrical path. Stainless steel that stays strong for years in dry air can fail much faster when exposed to water, salts, or stray electrical currents.
Fundamentals of Electrolytic Corrosion
Electrolytic corrosion works much like a simple battery. It needs a metal surface, an electrolyte, and a path for electrical current. When all three are present, material shifts from one spot to another. This process follows clear rules, but in stainless steel, it often leads to sudden failures if conditions are not controlled.
Corrosion happens because of electrochemical reactions. Stainless steel has spots that act as anodes and others that act as cathodes. At the anode, metal atoms lose electrons and turn into ions that dissolve into the electrolyte. At the cathode, electrons are used up by reactions such as oxygen reduction.
In normal conditions, the chromium oxide layer on stainless steel protects it from these reactions. But if that layer is damaged or an electrical current flows through the surface, the protection breaks down. The exposed areas start to pit, crack, or wear away. Once this damage begins, it often spreads because the weak spots have less protection than the rest of the surface.
Role of Electrolytes and Conductive Paths
Electrolytic corrosion needs an electrolyte to start. Moisture with salts, chlorides, or other charged particles makes a conductive solution that lets current flow. Examples include seawater, cleaning solutions, or condensation with airborne particles. The higher the ion level, the faster the corrosion moves.
A conductive path is also required to complete the circuit. This can form when stainless steel touches another metal or when stray electrical currents pass through a structure. Welds, fasteners, and joints made from mixed metals are common weak points. Once the path exists, the stainless steel becomes part of an electrochemical cell, and corrosion speeds up.
Difference Between General Corrosion and Electrolytic Corrosion
General corrosion spreads evenly across a surface. It often shows up as uniform thinning or discoloration. This type is easier to predict and manage because coatings or better material choices can slow it down.
Electrolytic corrosion is different. It is local, focused, and often more aggressive. It can create deep pits or cracks while leaving most of the surface untouched. Because it is uneven, it is harder to notice early. A part may look fine from the outside but be badly weakened inside.
Causes of Electrolytic Corrosion Stainless Steel
Electrolytic corrosion does not happen randomly. It appears when certain conditions allow current to flow and remove metal. These conditions often come from design choices, the working environment, or outside electrical factors.
Galvanic Coupling with Dissimilar Metals
When stainless steel touches a different metal in the presence of an electrolyte, a galvanic cell forms because the two metals have different electrical potentials; one acts as the anode and corrodes faster, while the other acts as the cathode and is protected.
For example, a substantial potential difference develops if stainless steel is fastened with carbon steel bolts in a damp setting. The weaker metal corrodes first, but the stainless steel can also suffer local damage near the contact points.
Stray Currents in Electrical Systems
Stray electrical currents are another major cause. These currents often come from poor grounding, nearby equipment, or electric rail systems. When current flows through stainless steel, it damages the passive film and speeds up corrosion.
Unlike galvanic corrosion, which needs two metals, stray current corrosion can affect stainless steel. Welds, joints, and stressed areas usually fail first, since the current concentrates on those spots.
Environmental Factors
Moisture provides the conductive path needed for electrolytic corrosion. When salts or acids are present, the reaction moves faster. Chloride ions are especially aggressive and can easily break through the protective film.
Marine settings, chemical plants, and food processing facilities often create these conditions. Stainless steel exposed to seawater, cleaning solutions, or acidic chemicals loses resistance quickly. The longer the exposure, the deeper and more damaging the corrosion becomes.
Mechanical Stress and Surface Damage
Mechanical stress makes stainless steel more prone to attack. Processes like bending, welding, or machining can stretch or disturb the protective film, leaving bare spots open to corrosion.
Surface damage from scratches, dents, or poor finishing also creates starting points for corrosion. Once the film breaks, electrolytic action begins more easily, especially when moisture or stray currents are present.
How to Identify Electrolytic Corrosion?
Electrolytic corrosion can be problematic to detect in the early stages. It does not always spread evenly, and the surface may look fine while damage develops underneath. Engineers and maintenance teams must spot the warning signs early to prevent failures.
One common sign is localized pitting, which shows up as small holes or cavities on the metal. These pits may form in clusters and grow deeper with time. Another warning sign is discoloration. This can look like dark spots, streaks, or uneven patches where the protective film has broken down.
Cracks or thinning in stressed areas, such as welds or bends, are also strong indicators. In some cases, parts may weaken without clear surface changes. Testing methods are used to find this hidden damage. These include electrical resistance checks, potential measurements, and non-destructive inspections, such as ultrasonic testing.

Prevention Strategies
Preventing electrolytic corrosion in stainless steel starts with proper planning. Choosing the right materials, designing carefully, and protecting surfaces all help lower risks and extend service life.
Proper Material Selection and Alloy Grades
Using stainless steel with higher levels of chromium, nickel, or molybdenum improves resistance. Selecting metals that are compatible in mixed assemblies avoids galvanic reactions. In harsh environments, duplex or marine-grade alloys provide longer-lasting durability.
Protective Coatings and Surface Treatments
Coatings and treatments add a barrier between stainless steel and its surroundings. Options include epoxy paints, corrosion-resistant films, or passivation treatments that strengthen the chromium oxide layer. Regular checks and maintenance keep these protections effective.
Design Considerations to Reduce Corrosion Risks
Good design helps prevent corrosion from starting. Avoid sharp corners, tight crevices, and rough welds where moisture can collect. Allow proper drainage and keep space between dissimilar metals to reduce galvanic contact.
Electrical Insulation and Cathodic Protection
Separating stainless steel from other metals with non-conductive materials stops galvanic corrosion. In high-risk settings, sacrificial anodes or cathodic protection systems can carry away stray electrical currents. This approach reduces material loss and increases service life.
Practical Solutions for Existing Corrosion
Once electrolytic corrosion begins, quick action is essential to stop it from spreading and to restore performance. The right solution depends on how deep the damage is and how critical the part is in service.
Mechanical cleaning is often the first step. Grinding, polishing, or abrasive blasting can remove surface corrosion and create a clean base. After cleaning, passivation treatments help rebuild the protective chromium oxide layer, giving the surface better resistance.
For more bottomless pits or cracks, repair welding may be necessary. Using the right filler metals and proper post-weld treatments prevents new corrosion from forming. Replacing the section may be the only safe option if the damage is too severe, especially when strength is affected.
Protective coatings are another practical method. Paints, epoxy layers, or membrane corrosion-resistant films form a barrier between stainless steel and its environment. Electrical insulation can also prevent galvanic action when stainless steel is near other metals.
When stray currents cause the problem, fixing grounding systems or adding sacrificial anodes can redirect the flow of electricity away from the steel surface. Regular inspections and ongoing maintenance are essential to keep repairs effective and extend the part’s service life.
Conclusion
Electrolytic corrosion in stainless steel occurs when electrical currents, moisture, and conductive paths break down the protective chromium oxide layer. It often begins where stainless steel touches other metals, faces mechanical stress, or comes into contact with salts and acids. Prevention requires wise material choices, careful design, protective coatings, and routine maintenance.
If you want to keep your stainless steel components safe or need solutions for existing corrosion, contact our team today for expert guidance and reliable support for your projects.
Hey, I'm Kevin Lee

For the past 10 years, I’ve been immersed in various forms of sheet metal fabrication, sharing cool insights here from my experiences across diverse workshops.
Get in touch

Kevin Lee
I have over ten years of professional experience in sheet metal fabrication, specializing in laser cutting, bending, welding, and surface treatment techniques. As the Technical Director at Shengen, I am committed to solving complex manufacturing challenges and driving innovation and quality in each project.