
Setup Cost vs Unit Cost in Sheet Metal Manufacturing
Every project starts with a question: why does the first piece cost so much more than the rest? This is a common frustration in sheet
We regularly update articles related to the manufacturing industry.

Every project starts with a question: why does the first piece cost so much more than the rest? This is a common frustration in sheet
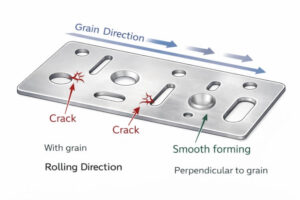
When holes, bends, or embosses sit too close together, the forming quality drops quickly. The metal cannot stretch evenly, which leads to cracks, wrinkles, or

Every electrical enclosure, control box, or metal assembly depends on clean and secure cable routing. Poor cable paths can lead to tangled wires, overheating, or
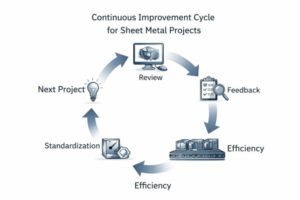
Design teams often run into delays or quality problems because each group works alone. Engineers may finish drawings without checking with manufacturing, and buyers may
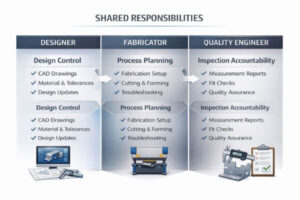
Modern sheet metal projects usually involve several teams — engineers, fabricators, suppliers, and clients. Problems start when their roles are not clearly defined. Even a

At first glance, a low quote feels like a victory. You save money, hit your budget, and move production forward. But months later, that same

A fair comparison requires uncovering what’s behind each quote. You need to check whether vendors use the same assumptions for material type, process selection, and inspection scope. Without that, comparing prices alone can be misleading and may affect your project’s budget or delivery schedule.

The key is to pay attention to how suppliers communicate, what details they include, and what they leave out. A professional quote should be clear, specific, and backed by technical understanding. Vague or overly simple quotes often signal inexperience or shortcuts that can affect your final parts.

Servo presses utilise sensors, control systems, and mechanical safety components to prevent damage before it occurs. They track force and position in real-time and stop the ram immediately if it exceeds the preset limit. This smart protection keeps tools in good shape, helps parts stay accurate, and supports smooth operation even when the load changes.
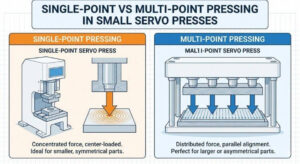
In precision forming, even small changes in pressing methods can affect the final result. Many engineers struggle to choose between single-point and multi-point pressing for

Light assembly work — such as connectors, sensors, and compact electronic housings — is getting more demanding every year. Manufacturers now need micron-level precision, repeatable

A safe small servo press system has several layers of protection. These include physical guards, sensors, interlocks, and emergency stop buttons. Each part works together to stop accidents, detect unusual conditions, and protect both the operator and the machine. When a system is designed with safety in mind, it stays reliable and reduces downtime.

If your press-fit process requires steady force, controlled speed, or live quality feedback, a servo press is worth considering. It lets you set pressing parameters digitally and achieve repeatability within microns. For delicate parts such as connectors, sensors, or housings, servo presses deliver consistent results while cutting down on rework and material waste.

In many precision-based applications, the answer is yes. Small servo presses provide programmable motion, quiet operation, and force accuracy within ±0.005 mm. They work well in industries like electronics, medical devices, and small assembly lines. However, hydraulic presses still hold an edge in heavy forming, deep drawing, and large stamping work.

A small servo press uses an electric servo motor to control motion, force, and position with great accuracy. Instead of relying on oil or air, it uses programmable electronic control. This gives users better flexibility and repeatable results. It is well-suited for industries that require precision, data tracking, and consistent force control.

Manufacturers today need to make parts that are smaller, lighter, and more accurate than before. Traditional mechanical and pneumatic presses often lack the control needed
We will contact you within 1 working day, please pay attention to the email with the suffix”@goodsheetmetal.com”